22-Jan-2024
| Chinese name | 硫脲 | English name | Thiourea |
|---|---|---|---|
| Russian name | Тиомочевина | Molecular weight | 76.14 |
| Molecular formula | CH₄N₂S | Номер CAS | 62-56-6 |
| Номер UN | 3077 | Номер HS | 293090 |
| Physical | Light yellow solid with a melting point of 176-178 °C and a density of 1.405 g/cm³. Soluble in water and alcohol-based organic solvents, it can be used in a variety of solvents. | ||
| Production and manufacturing | China-SINHON-Thiourea manufacturer | ||
| Precautions | Stable at room temperature, but sensitive to humid air. When the solution is heated, it is hydrolyzed to produce ammonia, carbon dioxide, and liquid H2S. Its solid powder is foul-smelling, toxic and should be used in a fume hood. | ||
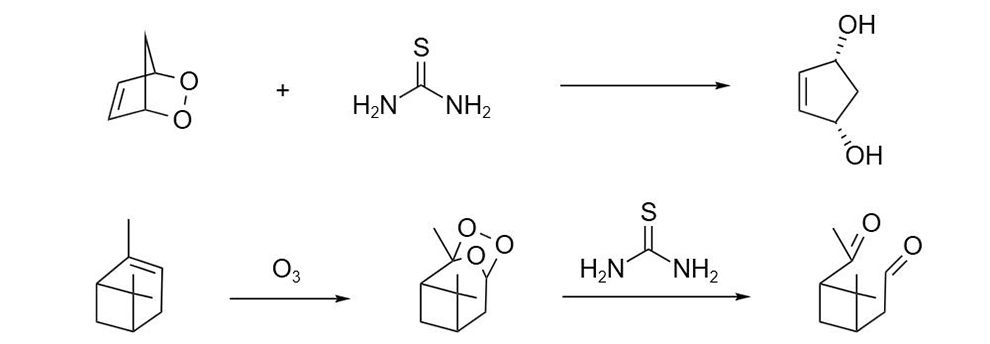
Thiourea is a sulfur-containing reagent and reducing agent commonly used in organic synthesis, mainly used for the reduction of internal peroxides and ozonions. Thiourea is often used in the synthesis of heterocyclic aminothiazole, pyrimidine and thihetacyclopropane and formamidine sulfinic acid, etc., and can also be used as an equivalent of nucleophilic hydrogen sulfide for the synthesis of acid chloride, sulfonic acid, mercaptan, sulfinic acid, etc.
Thiourea reagents can reduce peroxides to alcohols, e.g. the internal peroxides of cyclopentadiene to the corresponding glycols. It is also possible to reduce the ozonization obtained after the ozonation of olefins to the corresponding dicarbonyl compounds. This is an important method for converting olefins into carbonyl compounds, and it is also an important method for early degradation of olefins.
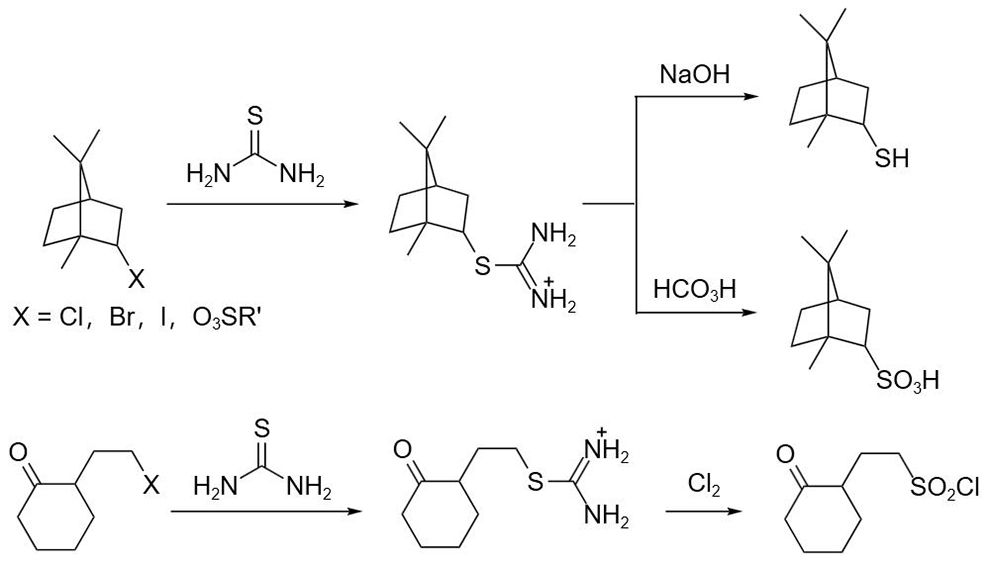
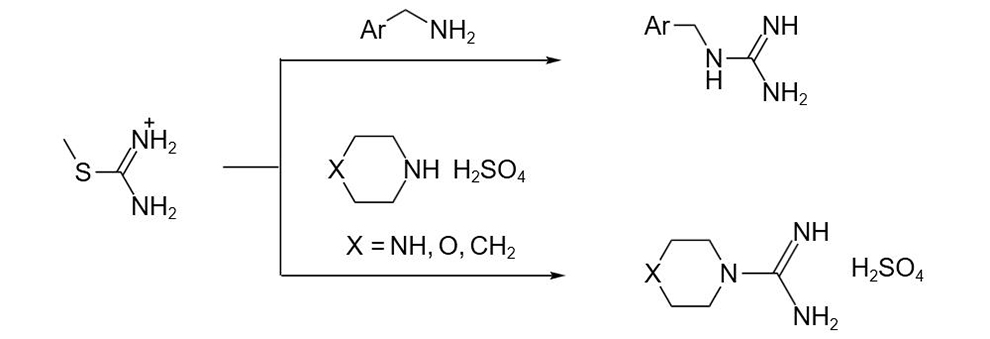
Another important use of this reagent is as an equivalent of hydrogen sulfide to prepare mercaptans, sulfonic acids and their acyl chloride. The ammonium isothiourea salt intermediate is obtained by reacting the sulfur atom in thiourea with alkyl halide or sulfonate, and the mercaptan can be obtained by hydrolysis under alkaline conditions. Compared with the method of preparing hydrophobic alcohols with hydrogen sulfide or hydrocarbons, this method has the advantage of avoiding the formation of by-product thioether, and many conditions can affect the conversion of ammonium isothiourea salt intermediates, such as: sulfonic acid can be obtained by peracid oxidation, sulfonyl chloride can be obtained by chlorine oxidation, and substituted guanidine can be obtained by replacing the alkyl sulfur group with amine.
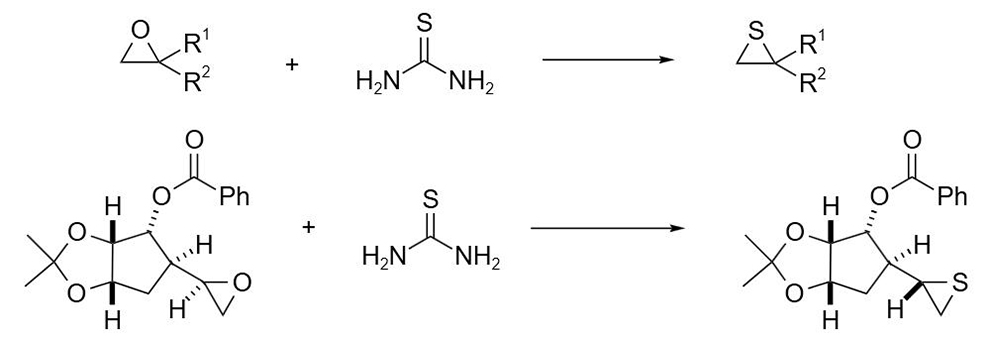
Thiourea can also be used to synthesize sulfur-containing atoms or heterocyclic compounds containing sulfhydryl groups. For example, thiourea reacts with epoxy compounds to convert epoxy compounds into corresponding thiheterocyclopropane derivatives in a single step. If chiral epoxy compounds are used, thiohetacyclopropane with inverted configuration can be obtained.
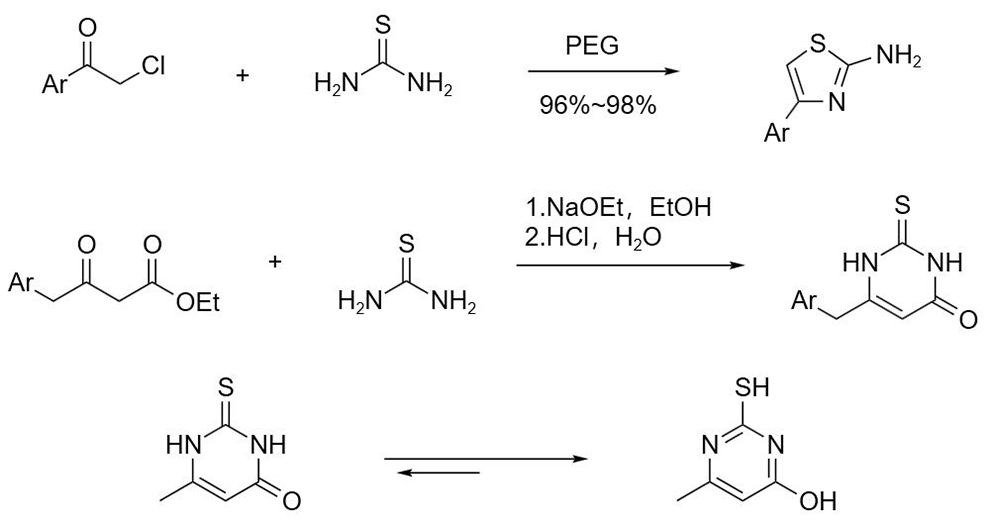
Thiourea reacts with α-haloketone to prepare 2-aminothiazole derivatives, and with β-oxocarboxylate to give 2,3-dihydro-2-thio-pyrimidin-4(1H)-one. The latter is prone to tautomerism to form 2-mercapto-4-hydroxypyrimidine.
Excerpt from: "Modern Organic Synthesis Reagents - Reductive Reagents"