17-Mar-2024
Chemical formula
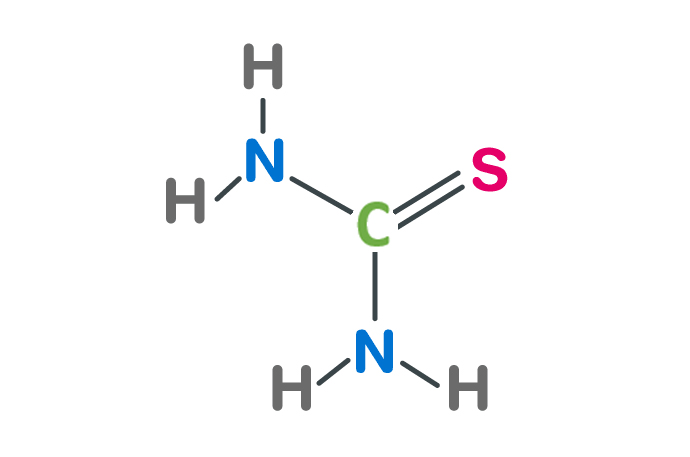
The chemical formula for thiourea is CH4N2S. It consists of one carbon atom (C), two nitrogen atoms (N), one sulfur atom (S), and four hydrogen atoms (H).
In the structure
In the structure, the central carbon atom (C) is bonded to two nitrogen atoms (N) and one sulfur atom (S).
Each nitrogen atom is bonded to one hydrogen atom (H).
The sulfur atom is also bonded to one hydrogen atom.
This arrangement results in a molecular structure where the carbon atom is at the center, with the nitrogen and sulfur atoms bonded to it. Each nitrogen atom has one hydrogen atom bonded to it, and the sulfur atom has one hydrogen atom bonded to it as well.
Molecular weight
To calculate the molecular weight of thiourea (CH4N2S), you'll need to sum the atomic weights of all the atoms present in the molecule.
Carbon (C): Atomic weight = 12.01 g/mol
Hydrogen (H): Atomic weight = 1.008 g/mol
Nitrogen (N): Atomic weight = 14.01 g/mol
Sulfur (S): Atomic weight = 32.07 g/mol
Now, let's sum these atomic weights, considering the molecular formula CH4N2S:
Molecular weight = (12.01×1) + (1.008×4) + (14.01×2) + (32.07×1)
=12.01+4.032+28.02+32.07
=76.132g/mol
So, the molecular weight of thiourea is approximately 76.132g/mol.
--END--
Sinhon Chemical focuses on high-quality thiourea production and manufacturing. The thiourea we produce has high purity, good quality and stable product performance. The annual production capacity is 20,000 tons, the inventory is sufficient, and the supply is stable. We also trade other products, such as molecular sieves, silica gel desiccant, aniline, methylene chloride, chloroform, sodium hydroxide, propylene oxide, etc.
Excellent service, reliable quality, delivering more value.
Contact us to start our journey of cooperation.
Email: [email protected]