Thiourea is a sulfur-containing reagent and reducing agent commonly used in organic synthesis, mainly used for the reduction of internal peroxides and ozonions.

where R1 is a hydrocarbon group or a substituted hydrocarbon group, and R2 is a hydrogen (H), hydrocarbon group or substituted hydrocarbon group.

The preparation of thiourea dioxide belongs to the field of peroxide preparation technology in organic chemistry, which is characterized by adding water in the reactor in advance, and then putting the raw material thiourea in batches, and adding hydrogen peroxide solution containing stabilizer (commonly known as hydrogen peroxide) at the same time to prepare thiourea dioxide.
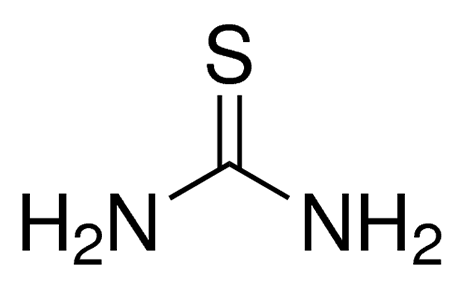
Thiourea, also known as urea sulfate, thiourea trioxide, urea sulfate, etc., is a compound containing sulfur, nitrogen, and oxygen. Its main uses include dyes, drugs, pesticides, oils, pulp, etc. With the advancement of technology and the expansion of the scope of application, the thiourea industry is experiencing a stage of rapid development.

Thiourea is an organic compound with the chemical formula CH4N2S. It is structurally similar to urea but with sulfur instead of oxygen in the backbone. Thiourea is a white crystalline solid at room temperature, soluble in water, and has a characteristic odor similar to that of ammonia.