
Thiourea, a sulfur-containing compound, has several applications in the pharmaceutical industry:

Thiourea, a compound with the chemical formula (NH₂)₂CS, has long captured the interest of researchers and industries alike due to its diverse array of applications across various sectors. High purity thiourea, in particular, has emerged as a valuable commodity, finding its place in industries ranging from agriculture to pharmaceuticals. Let's delve into the world of high purity thiourea, exploring its properties, applications, and recent advancements.
Thiourea organocatalysis involves the use of ureas and thioureas to accelerate and stereochemically alter organic transformations. Unlike classical catalysts, these organocatalysts interact through non-covalent interactions, particularly hydrogen bonding. Here are some key points:

Thiourea derivatives are increasingly recognized for their ability to act as halogen bond donors, contributing to the field of supramolecular chemistry. Halogen bonding involves the formation of attractive interactions between halogen atoms (typically iodine, bromine, or chlorine) and Lewis bases. Thiourea derivatives are particularly interesting because of the electron-deficient nature of the sulfur atom and the potential for nitrogen atoms to serve as hydrogen bond acceptors. Here's a detailed description of thiourea halogen bond donors:
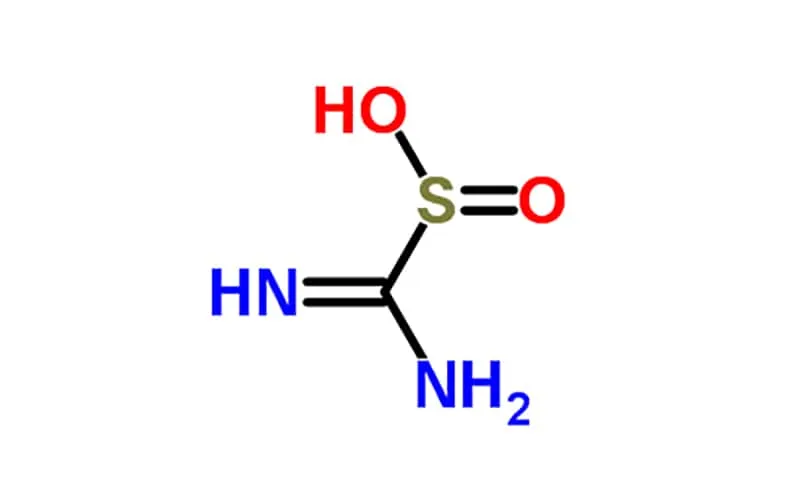
Thiourea and thiourea dioxide are two distinct chemical compounds with different properties and applications:

Product Name:THIOUREA CAS Number: 62-56-6