
Thiourea can be used in metal processing, textile industry, photography, organic synthesis, chemical analysis, electroplating, medicine, dye sensitization and other fields. Due to its unique properties, thiourea finds applications in various industrial and scientific fields.

Thiourea and ethylene thiourea (ETU) are both chemical compounds containing the thiourea functional group, which consists of a sulfur atom doubly bonded to a nitrogen atom and single-bonded to another nitrogen atom. However, they have different molecular structures and properties.

Thiourea, a bitter taste, is a compound with the chemical formula (NH2)2CS, is not typically consumed because it is toxic to humans. In fact, ingesting thiourea can be extremely dangerous and is not recommended under any circumstances.

Urea and thiourea are both organic compounds containing nitrogen, carbon, oxygen, and hydrogen atoms, but they differ in their chemical structures and properties: 1.Chemical Structure: Urea: Urea has the chemical formula CO(NH2)2.
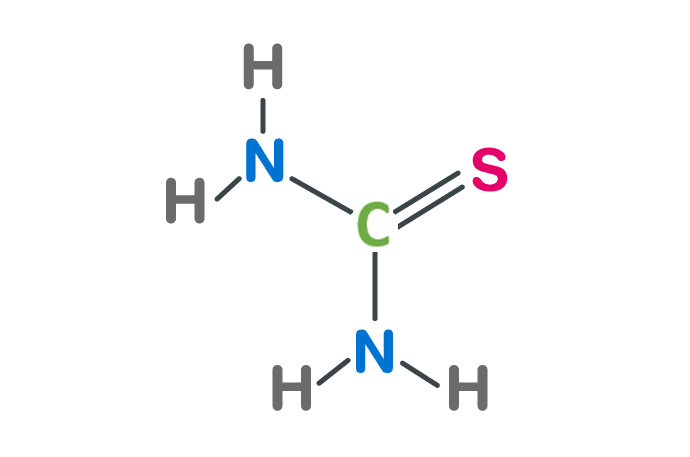
The chemical formula of thiourea is CH4N2S, and its molecular weight is approximately 76.132g/mol. It consists of 1 carbon atom (C), 2 nitrogen atoms (N), 1 sulfur atom (S) and 4 hydrogen atoms (H). This arrangement forms a carbon atom in the center, nitrogen atom and sulfur atom. The molecular structure to which it binds.
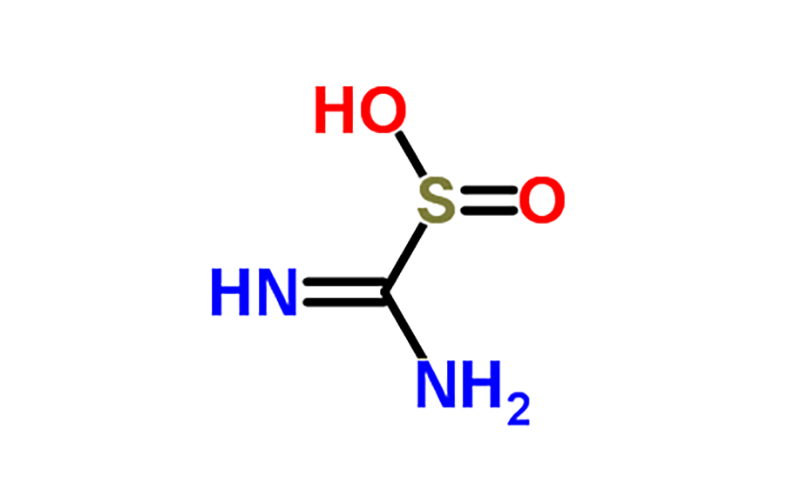
Thiourea dioxide, also known as formamidine sulfinic acid, is an organosulfur compound derived from thiourea. It is commonly used as a reducing agent in various industrial processes, including textile bleaching and paper production. Thiourea dioxide is produced by the reaction of thiourea with hydrogen peroxide under alkaline conditions.